Biosimilars Business
What is Biosimilars?
It is called as a biosimilar that has the same efficacy and safety with new bio medicine guaranteed by the authority.
Characteristics of biosimilar
Similar but not the same.
Unlike the generic medicines, bio medicines can not be produced exactly same as their predecessors.
The main raw materials used for developing bio medicines are those in which organisms produced such as from Escherichia coli or cultured cells. Like a human being which has many differences between individuals, cells produced from the same organism are not exactly the same. It is impossible to create a biosimilar product with exactly the same structure as the new bio medicines.
Same efficacy and safety as the new bio medicines
In order to be approved as a biosimilar, it is required to prove that the efficacy and safety of the drug are the same as those of new bio medicines.
Compared to genetic medicines, bio medicines have a huge and complex molecular structure, which results in a huge development period and cost.
Also, as described above, it is not possible to produce a product with exactly the same structure, therefore the same level of clinical trial is required as new medicines.
About the approval of biosimilar products
A generic medicine does not require a clinical trial if it has the same structure as that of the originals, but a biosimilar requires same level of clinical trial with a new medicine.
As a result, it is possible to prove the efficacy and safety.
-
New Drugs
In the application form for new drugs, seven kinds of materials for manufacturing methods, quality, and clinical trials must be submitted.
-
Generic Medicine

In the case of generic medicine, approx. submissions are completed only with about three kinds of materials.
-
Biosimilars
In the application form of biosimilars, it is required to submit almost the same level of materials as the new medicine.
Social significance of biosimilars
-
Present status
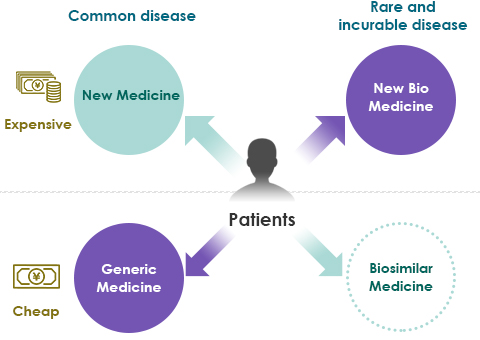
- Use of biologics produces high medical costs.
- Even as generics are adopted, the use of expensive biologics also increases, and society as a whole does not see a large reduction in medical costs.
-
Ideal
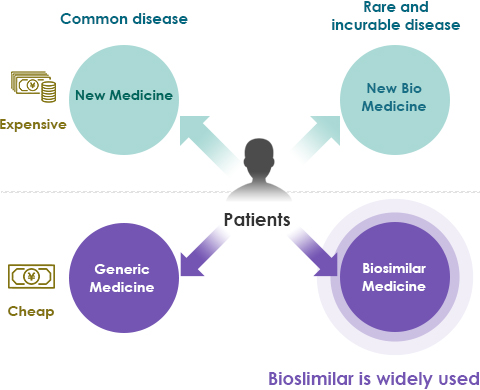
- Lower medical costs
- Lower medical costs mean that more patients can receive advanced care.
- Reduces the strain of healthcare financing on the Japanese government

Developing biosimilars to supply medicine with high quality to many patients as possible